



Technical Basics Part 6
Cells and power supplies
2J1 Understand that a battery is a combination of cells (usually in
series).
A battery is a combination of cells usually in series and provides a potential difference (a voltage) at its terminals. One terminal is marked POSITIVE and the other NEGATIVE and the battery will provide DC DIRECT CURRENT when connected into a circuit.

Up to this moment you probably thought that the picture above shows a battery. In terms of going into a shop and asking for a battery you would still be right, but for the Foundation course ( and to be technically correct for all Amateur Radio) you have to know that the picture above shows a single cell and that a battery is a number of cell either linked in series or parallel.
 What
does series and parallel mean ? Ok I will come back to that in a minute.
What
does series and parallel mean ? Ok I will come back to that in a minute.
First let me tell you about a cell. One end is marked + and is the "positive" end and the other is often but not always marked - and is the "negative" end. The positive end is often the one with a protruding pip.
It is from the negative end that electrons are in abundance and they are all trying to reach the positive end. If you were to link a wire from the negative end to the positive end then electrons would flow. What you would have created is what is called a "CIRCUIT" between the positive and the negative terminals
It would not do any good purpose except what we call flatten the battery, when all the spare electrons have flowed down the wire to the positive end. Even with a battery shown above if the wire is not thick enough it could get hot and thus burn your fingers.
NEVER connect a wire from the positive terminal to the negative terminal of any cell or battery else a fire could result. Such connection is called a "short circuit".
Recall that a battery provides electrical energy from stored chemical energy and has a Potential Difference across its terminals.
In the battery we have said that there is an abundance of electrons trying to leave the negative terminal and reach the positive terminal. We could also say that there is an opportunity for a current to flow if a wire was connected (or the "potential" for a current to flow if a wire was connected). The word potential is taken further in electronics and added to the word "difference" and we say that there is a POTENTIAL DIFFERENCE between the negative and positive terminals and the greater the number of electrons trying to make the journey the larger the potential.
The potential difference is measured in volts. Thus it is also said that a battery supplies a voltage.
Confusing Eh!
Series and parallel
We are going to digress from the syllabus for a
moment as it is important for you to understand the concept of what
electronic components linked in parallel and alternatively linked in
series means .......
Linked in Parallel
 What does series and parallel mean? It is the way
batteries can be connected together. Let us explain further below.
What does series and parallel mean? It is the way
batteries can be connected together. Let us explain further below.
You are now aware that a cell has two terminals one + and one -.
If we had three cells and joined all the + together and then joined all the - together we would have the cells in parallel. See diagram below.
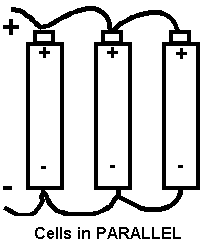
Remember that a combination of 2 or more cells forms a battery.
Linked in Series
If we had three cells and joined the - of one cell to the + of the next and the - of that cell to the + of the third cell the cells would be arranged in series.
You might like to remember that linked in SERIES is just like a STRING of SAUSAGES you can buy at the supermarket;- each join only to the next one.
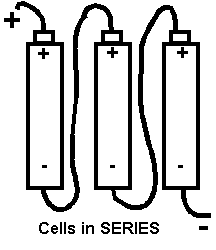
Remember that a combination of 2 or more cells forms a battery.
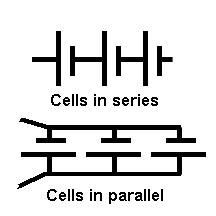
CIRCUIT DIAGRAM SYMBOLS
These circuit diagram symbols will be introduced to you bit by bit (as necessary).
The two drawings above are representations of parallel and series batteries but they took a great number of lines to draw and there is an easier way by the use of what are called "circuit diagrams symbols" to represent the batteries.
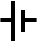
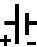
Above is the circuit symbol of a cell and the second one is annotated to show the positive and negative terminals. In circuit diagrams the annotation is not used but is given here for you to learn. The positive side is the longer of the two lines.
The batteries shown above could therefore have been drawn as follows:-
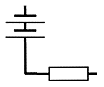
A circuit is needed to allow current to flow.
To make a circuit we need several items :-
For there to be a circuit in which current will flow there must be an unbroken path between one battery terminal and the other.
What does this circuit have ?
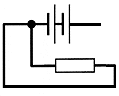
 |
Battery Yes Resistance Yes Wire links Yes Links from one side of battery to another No - thus not a complete circuit in which electron (current) can flow. |
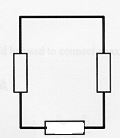
 |
Battery Yes Resistance Yes Wire links Yes Links from one side of battery to another No - thus not a complete circuit in which electron (current) can flow. |
 |
Battery No Resistance Yes Wire links Yes No battery thus no source of electrons thus not a complete circuit in which electron (current) can flow. |
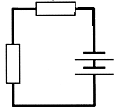 |
Battery Yes Resistance Yes Wire links Yes Links from one side of battery to another Yes - thus a complete circuit in which electron (current) can flow. |
|
The resistance could just have easily have been a light bulb 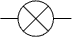 when this circuit symbol would have been used in place of the resistor |
Well not if you take if slowly, so here is a re-cap and if there is anything here you do not understand stop and re-read as to progress from here will not make much sense until you have a good grasp of what is written above.
Recall that any unwanted battery must be properly
disposed of.
There are two types of batteries a Primary Battery and a Secondary battery. The Primary battery is a battery with several cells in which a chemical process takes place until it is exhausted. This is also called the battery is "FLAT", then your battery of cells must be disposed of in the correct manner at your local waste recycling centre if they are a large battery, but smaller batteries can often be disposed of in recycling bins provided by stores or garden centres.
A Primary Battery cannot be and you must not try to recharge such a battery..
Understand that a rechargeable (secondary) battery has a reversible chemical process
Rechargeable batteries are marked as "rechargeable" and have a similar chemical process going on within them until they are nearly exhausted ( they must not be fully exhausted else they may not recharge ) but the chemical process can be reversed by attaching the rechargeable battery to a specially designed "charger" which will restore their voltage levels.
The origin of some of the text on this page is from the RSGB with additions by the web master